Authors: Emad N. El Qada
DOI: https://doi.org/10.48103/jjeci2122019
JORDANIAN JOURNAL OF ENGINEERING AND CHEMICAL INDUSTRIES (JJECI)
Pages: 92-99
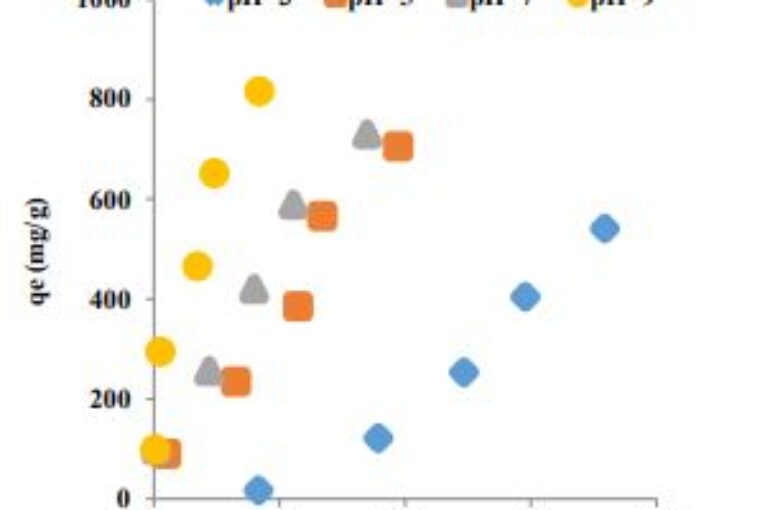
The focal theme of this work is to assess the ability of Jordanian diatomite to treat MG-bearing effluents. Effects of several experimental parameters namely, particle size of diatomite, pH and initial MG concentration were investigated through liquid-phase adsorption processes. Several equilibrium isotherm models were applied. It was found that initial MG concentration, pH and particle size of diatomite had a significant effect on the adsorption process. MG uptake has increased from 99.3 mg/dm3 to 898.7 mg/dm3 over the whole concentration range. A high percentage of MG removal (99.6%) was achieved as the diatomite particle size decreased from 500-710μm to 125-250μm. The optimum pH for the
removal of MG was=9. Freundlich model was satisfactorily applied to the experimental data.
Keywords: Adsorption, Equilibrium isotherm, Malachite green, Diatomite, Wastewater Treatment.