Authors: Aiman Al-Rawajfeh
DOI: https://doi.org/10.48103/jjeci1102018
JORDANIAN JOURNAL OF ENGINEERING AND CHEMICAL INDUSTRIES (JJECI)
Pages: 78-83
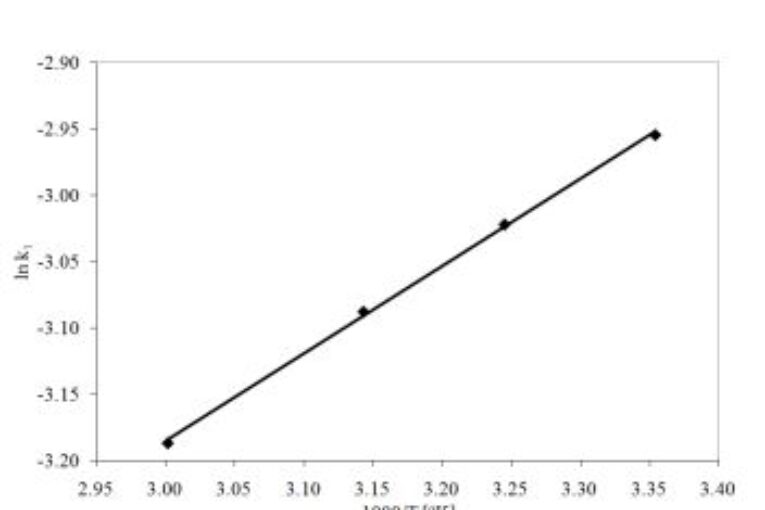
Adsorption is a widely used technique for the removal of pharmaceutical organic micro-pollutants. In this article, calcined gypsum (CaSO4.0.5H2O) was utilized for the removal of ibuprofen medicine from polluted water. Several factors including the adsorbent dose, contact time, and temperature were studied. The influence of the ions in the solution on the precipitation of gypsum and its setting time was investigated because it significantly affects the percentage removal. The fast the setting time gypsum, the lower the percentage removal precipitate. From thermodynamic parameters, the negative values of ΔGo indicated a spontaneous and physisorption of ibuprofen onto the calcined gypsum surface. Kinetic study results showed that the adsorption of ibuprofen on gypsum follows pseudo-first-order kinetics.
Keywords: Calcined Gypsum, Wastewater Treatment, Removal, Ibuprofen

![Different Polyoxometalate Structures Obtained from the Na11H[H(2-x)Bi2W20O70(HWO3)x]·46H2O(x=1.4).](https://jjeci.net/wp-content/uploads/2019/12/111244-165x109.jpg)