Authors: Hossam Al-Itawi
DOI: https://doi.org/10.48103/jjeci322020
JORDANIAN JOURNAL OF ENGINEERING AND CHEMICAL INDUSTRIES (JJECI)
Pages: 11-18
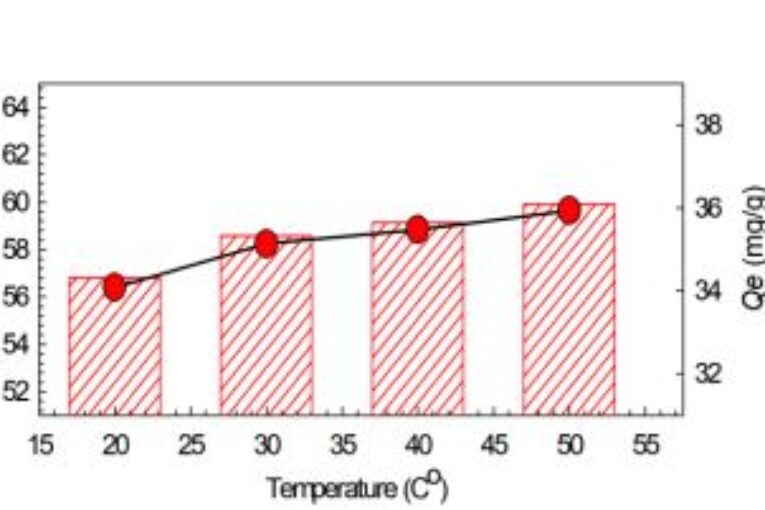
It has been established that the presence of paracetamol in wastewaters can cause a potential risk to the environment. This work examined the possibility of using calcined gypsum in removing paracetamol from aqueous solutions. At neutral pH conditions, calcined gypsum was successful in removing paracetamol via adsorption, from aqueous solutions with a removal efficiency that ranged between 56.8 to 65.3 % of an initial concentration of 600 ppm. Increased temperature (from 20 to 500C) had a minor effect on the removal % of paracetamol while increasing the initial calcined gypsum dose (from 0.5 gm to 3 gm)
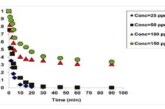
and contact time (up to 15 min) increased by the removal % of paracetamol. Thermodynamically, the adsorption of paracetamol by calcined gypsum process was found to be spontaneous and endothermic, and more likely a physical process, while kinetically; the Pseudo-Second order model was found to be the best fit compared to the Elovich model. The removal process mainly consists of two stages, and it could be deduced from the kinetic behavior of paracetamol adsorption that the recrystallization process can be another rate-limiting step in the process.
Keywords: Paracetamol, Calcined Gypsum, Adsorption, Kinetics Study, Pseudo-Second Order