Authors: Niveen Assaf, Ibrahim Suleiman, and Bassam Malkawi
DOI: https://doi.org/10.48103/jjeci712024
JORDANIAN JOURNAL OF ENGINEERING AND CHEMICAL INDUSTRIES (JJECI)
Pages: 1-4
Abstract
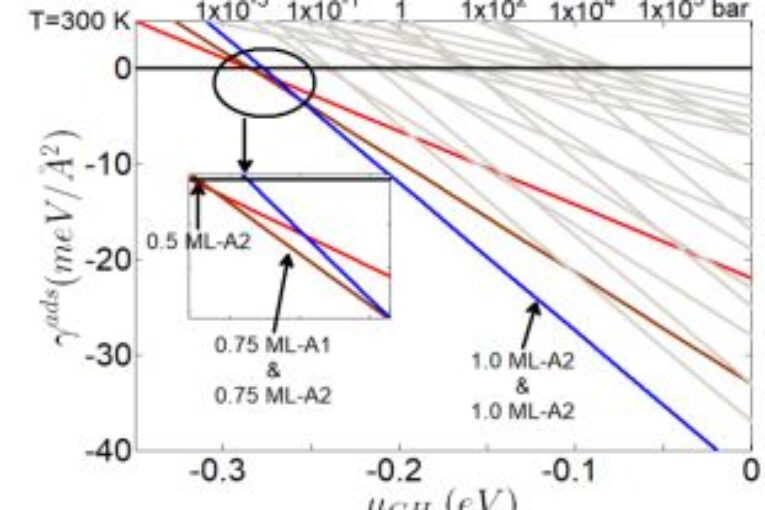
The present study utilized the ab initio atomistic thermodynamics technique to assess the stability of pure carbon dioxide and pure methane on the calcite(10.4) systems. The stability of configurations 0.5 ML-A2, 0.75 ML-A2, and 0.75 ML-A1 in CH4/calcite (10.4) systems was shown to be considerable, but only within a limited range of chemical potential. The 1.0 ML-A1 and 1.0 ML-A2 systems of CH4/calcite (10.4) demonstrated remarkable stability throughout a wide range of chemical potentials. The predominant stable forms for CO2/calcite (10.4) systems are the 1.0 ML-B2 and 1.0 ML-A4 structures. The surface free energy phase diagrams demonstrate that CO2is more favourable than CH4 for adsorption on the calcite (10.4) surface.
Paper type: Short communication paper
Keywords: EGR, atomistic thermodynamics, methane, carbon monoxide.
Citation: Assaf, N., Suleiman, I., and Malkawi, B.“ The Equilibrium Stability of CH4 and CO2 on the Calcite (10.4) Surface: An Atomistic Thermodynamics Investigation”, Jordanian Journal of Engineering and Chemical Industries, Vol. 7, No.1, pp: 1-4 (2024).