Authors: Alanood A. Alsarayreh
DOI:
JORDANIAN JOURNAL OF ENGINEERING AND CHEMICAL INDUSTRIES (JJECI)
Pages: 102-119

Abstract

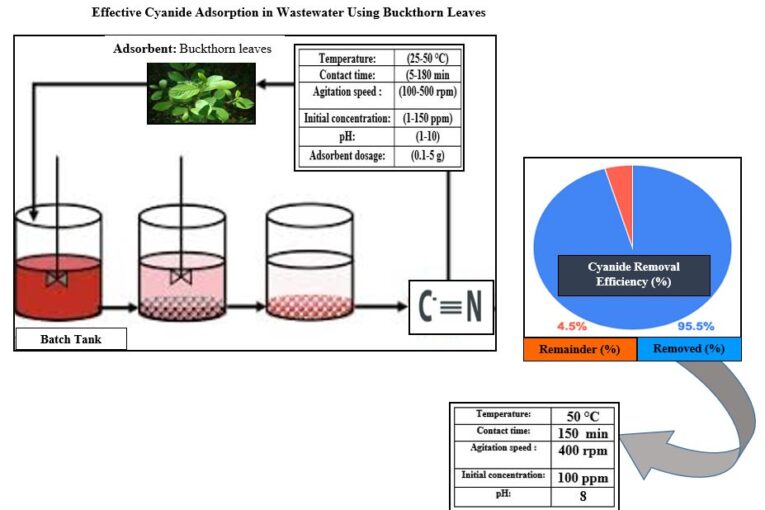
Cyanide is an extremely toxic compound that prevents cellular respiration by binding to cytochrome c oxidase, that causes a speedy oxygen deficiency and potentially fatal consequences for affected organisms. Therefore, the elimination of cyanide from wastewater is of pronounced health and environmental position. The current study focuses on investigating the removal of cyanide by adsorption practice by means of buckthorn leaves as low-cost and an available adsorption medium. The cyanide removal process is conducted in a batch mode unit and under different operating conditions of temperature (25-50 °C), contact time (5-180 min), agitation speed (100-500 rpm), initial concentration (1-150 ppm), pH (1-10), and adsorbent dosage (0.1-5 g). The obtained results show that the removal efficacy is proportional to all factors except the initial concentration, and that the highest cyanide recovery rate of 95.5% is attained at pH of 8, 400 rpm for agitation speed, initial concentration of 100 ppm, adsorbent dose of 4 g, contact time of 150 min, and 50 °C of temperature. According to the correlation coefficient, the isothermal study confirm that the Langmuir model is the closest to representing the experimental data with a value of 0.9994, slightly ahead of the Freundlich and Temkin models. Also, the pseudo-second-order model records to be the best in representing the data kinetically with a correlation coefficient of 0.9999, which is ahead of the pseudo-first-order model (the Elovitch model), and the intra-particle diffusion model. Thermodynamically, the adsorption is endothermic and positively entropic with values of 159 kJ/mol and 571.8 J/mol K, respectively, and spontaneous at all temperatures studied.
Paper type: Research paper
Keywords: Adsorption; Buckthorn leaves; Batch unit; Cyanide; Removal efficiency.
Citation: Alsarayreh.A, ” Effective Cyanide Adsorption in Wastewater Using Buckthorn Leaves: A study on Removal Efficiency and Kinetic Analysis..”, Jordanian Journal of Engineering and Chemical Industries, Vol. 8, No.1, pp: 102 – 119 (2025).